press
Press releases
October 12, 2023: Dry Eye Foundation Launches New Eye Drop Lookup Tool
July 25, 2023: Dry Eye Foundation Applauds Breakthrough Investigation of OTC Eye Drop Safety Issues
July 19, 2023: Dry Eye Foundation Launches Mermaid Tears
June 22, 2023: Dry Eye Foundation Notifies Authorities of over 200 Potentially Unsafe Eye Drops
April 14, 2023: Dry Eye Foundation Warns about Alarming Surge of Unsafe Eye Drops
-
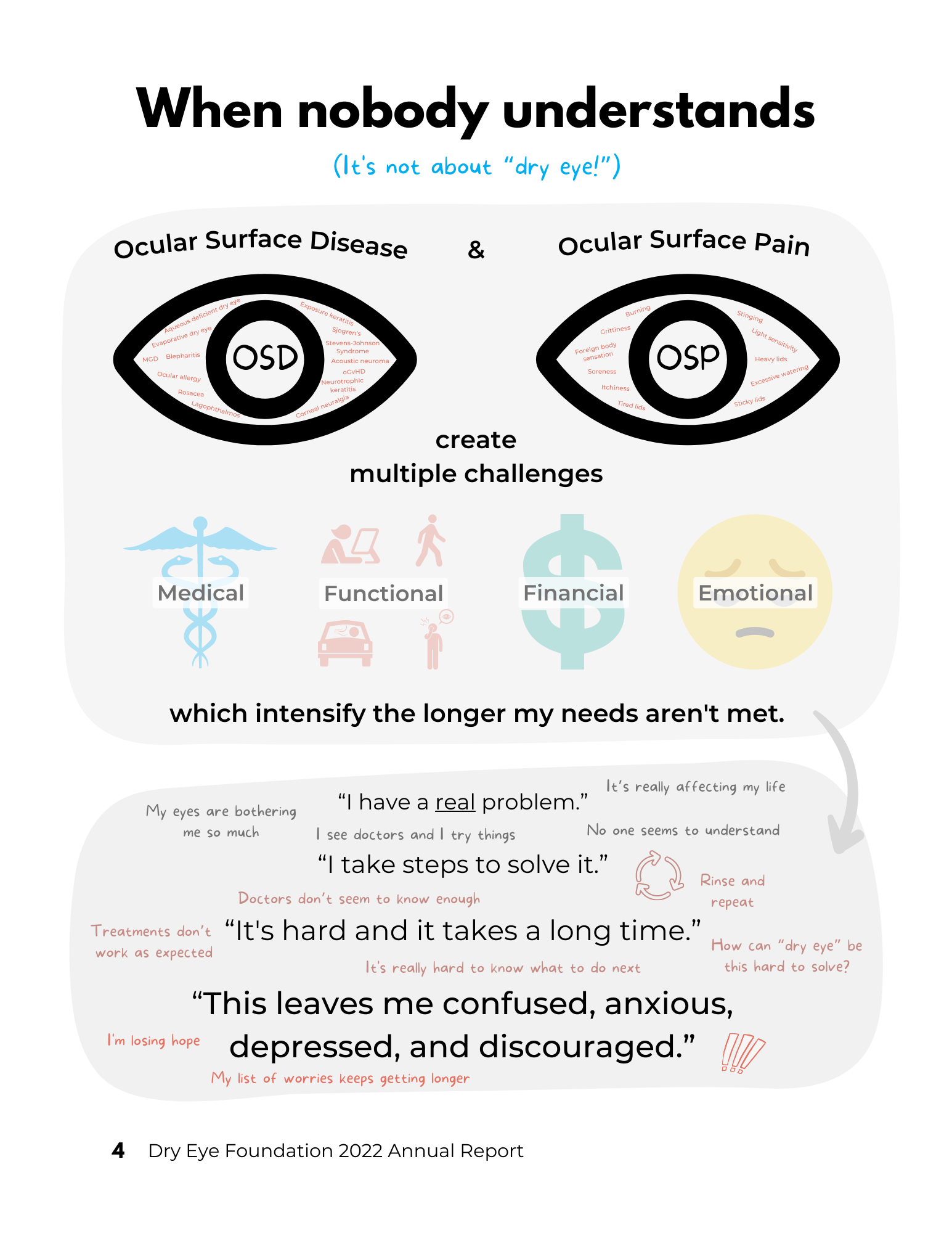
The Dry Eye Foundation is a 501(c)(3) nonprofit patient advocacy and education organization whose mission is to improve quality of life for people with ocular surface diseases.
DEF began informally in 2005 as the Dry Eye Zone, a popular patient-run online community and information portal, and in 2018 incorporated and received its 501(c)(3) status.
PROGRAMS:
COMMUNITY SERVICES: Dry Eye Helpline, Zoom support groups, social media groups, discussion forums and Dry Eye Happy Hour (see Community support)
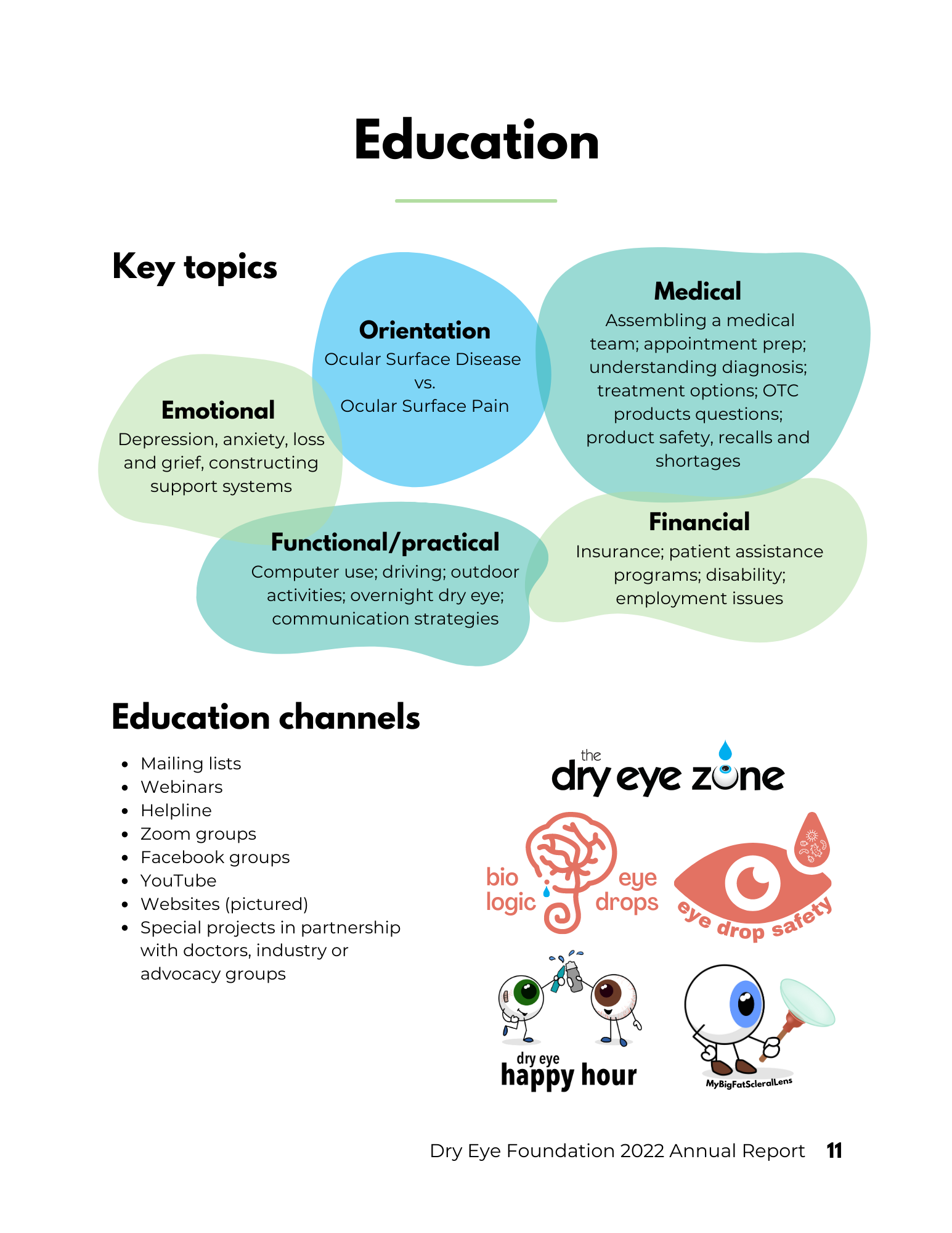
EDUCATION: websites eyedropsafety.org, biologiceyedrops.org, dryeyezone.com, dryeyestories.com, mydryeyedata.org, mybigfatsclerallens.com, in addition to social media and email education campaigns
RESEARCH: DEF collaborates with academic institutions and industry in designing and conducting patient-centered dryeyezone.com research. In 2020-2021, DEF conducted an exhaustive community survey which is used in our education and advocacy (see mydryeyedata.org).
ADVOCACY: DEF works to equip individuals to advocate for themselves, and to elevate the patient voice in eye care and in industry. In addition, from time to time, DEF chooses to engage a specific overlooked problem or need, dryeyezone.com particularly those related to patient safety.
Find us on Guidestar (Gold rating)
-
800-484-0244
Rebecca Petris (Poulsbo, WA) - Co-Executive Director
800-484-0244 x1
Rebecca has dry eye, neuropathic pain from LASIK, and founded Dry Eye Zone in 2005 (predecessor to DEF). Rebecca is a DEF co-founder, serving as President of the Board of Directors since 2018.
Projects: DryEyeTalk.com, DryEyeZone.com, MyDryEyeData.org, BiologicEyeDrops.org, EyeDropSafety,org, Community services
Sandra Brown, MD (Concord, NC) - Medical advisor
Dr. Brown is an ophthalmologist focusing on pediatric ophthalmology, adult strabismus, neuro-ophthalmology and forensic ophthalmology. She is an at-large board member.
Projects: BiologicEyeDrops.org, EyeDropSafety.org
Aidan Moore (Poulsbo, WA) - Co-Executive Director
800-484-0244 x2
Aidan has worked in the dry eye field for the past seven years. He is a DEF co-founder and has previously served as Secretary and Treasurer. He graduated from Pomona College in 2020.
Projects: DryEyeStories.org. MyDryEyeData.org, Community services
-
Associated Press, “After recalls and infections, experts say safer eyedrops will require new FDA powers” (December 13, 2023)
MedPage Today: “What to Know About Ongoing Eye Drop Recalls” (November 21, 2023)
New York Post: “Tainted eyedrops pulled by CVS, Target made by barefoot workers in India: report” (November 10, 2023)
Bloomberg: “Tainted Walmart, CVS Eyedrops Tied to Unsanitary Indian Factory” (November 9, 2023)
Vision Monday: “The Optical Community Weighs In on the Latest FDA Eye Drop Warning” (November 11, 2023)
Popsugar: “Are Eye Drops Still Safe to Use? Here's What the Latest FDA Recall Means” (November 2, 2023)
Optometry Times, “FDA waves red flag over eye drops for potential risk of eye infection” (November 2, 2023)
Vision Monday, “Dry Eye Foundation Introduces New Eye Drop Lookup Tool” (October 16, 2023)
Drugwatch, “Survey: Eye Drops Sold Online Pose Risks to Consumers” (August 10, 2023)
Bloomberg Businessweek, “No Testing, No Inspections: Contaminated Eyedrops Blinded and Killed Americans” (July 17, 2023)
Managed Healthcare Executive, “Over 200 Potentially Unsafe Eye Drops Reported to FDA” (June 23, 2023)
Vision Monday, “Dry Eye Foundation Reports Potentially Unsafe Eye Drops to FDA” (June 23, 2023)
Optometry Times, “Dry Eye Foundation takes a stance on eye drop safety, encourages consumer education” (June 2, 2023)
Statesman Journal, “Eyedrop safety: How to avoid unsafe products this allergy season” (May 16, 2023)
Ophthalmology Times, “Dry Eye Foundation continues to warn against the use of unverified and unsafe eye drops” (May 12, 2023)
Eyewire, “Dry Eye Foundation Calls on Retailers to Address Rampant Sales of Potentially Unsafe Eye Drops” (May 10, 2023)
-
-
YouTube playlist: Safety Alerts
YouTube playlist: Biologic Eye Drops
Open Letter to the Food and Drug Administration (May 12, 2023)
Open Letter to the Ophthalmic and Optometric Press (May 5, 2023)
Open letter to M2 Biologics LLC (March 9, 2023).
Open letter to Regenerative Processing Plant LLC (March 5, 2023).
Open letter to Regenerative Processing Plant LLC (September 29, 2022).
Regulatory Compliance Status of Certain Commercial Biologic Eye Drops (June 8, 2022).
ALERT: Eye drops containing proteins derived from human reproductive tissue or fluid and commercialized for treatment of ocular surface disease (May 16, 2022).
-
BEBRF Webinar: Managing Dry Eye (February 2023)
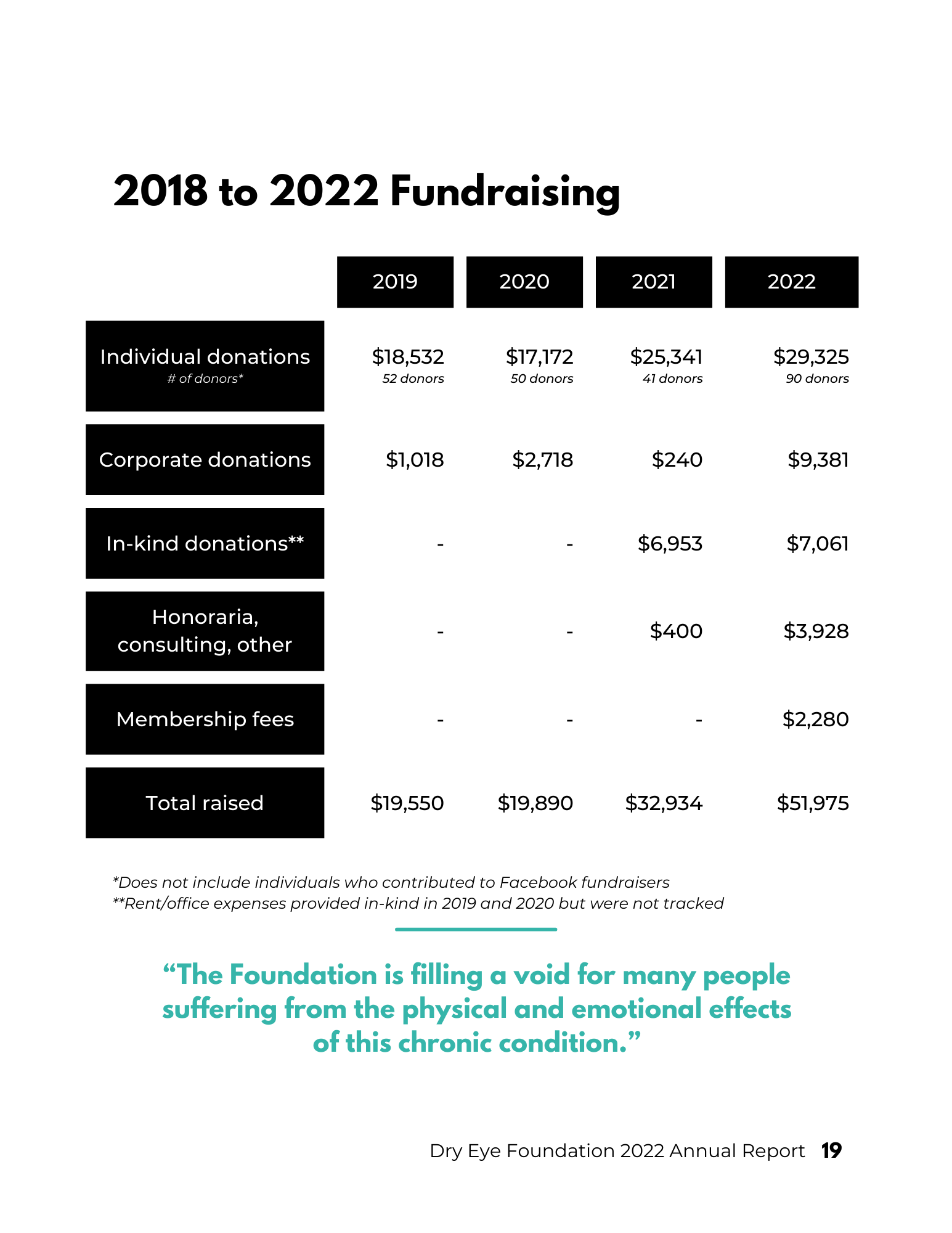
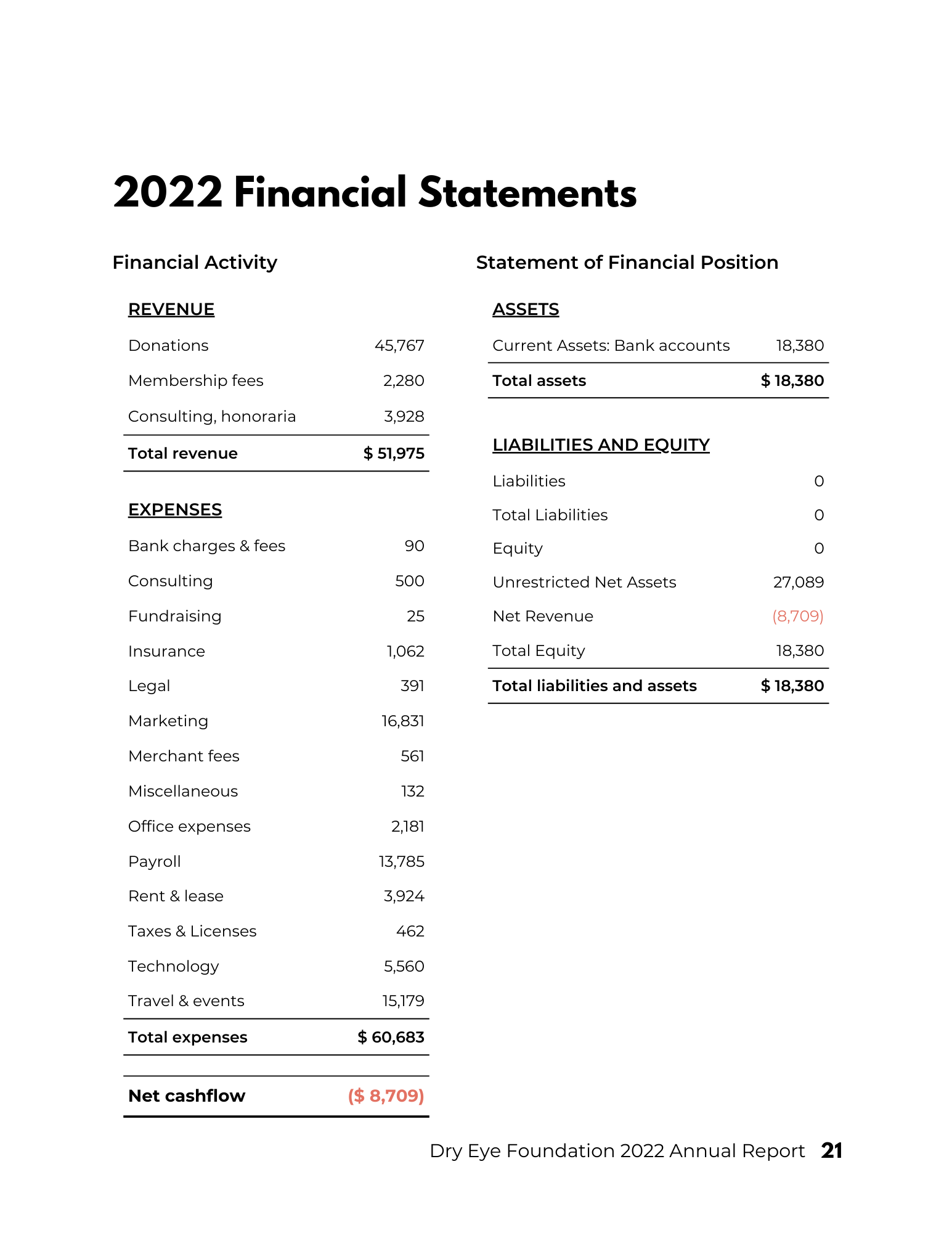
2022 Annual Report